Sbírka 111 Atom Structure Of Calcium
Sbírka 111 Atom Structure Of Calcium. The atomic number is always equal to the number of protons in the nucleus and the number of electrons (in an atom, not an ion) orbiting the nucleus. With a standard atomic weight of circa 1.008, hydrogen is the lightest element on the periodic table.
Nejlepší Calcium Atomic Structure Stock Image C023 2483 Science Photo Library
The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. It is also used as an alloying agent for aluminium, beryllium, copper, lead and magnesium alloys. Calcium appears as a silvery, soft metal that turns grayish white on exposure to air.(image to be added soon) number of energy levels:
The chemical symbol for hydrogen is h. The nucleus is composed of protons and neutrons. The atomic number is always equal to the number of protons in the nucleus and the number of electrons (in an atom, not an ion) orbiting the nucleus. (image to be added soon) number of energy levels: It is also used as an alloying agent for aluminium, beryllium, copper, lead and magnesium alloys. Calcium electron configuration notation the configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. The chemical symbol for hydrogen is h. Calcium metal is used as a reducing agent in preparing other metals such as thorium and uranium.

With a standard atomic weight of circa 1.008, hydrogen is the lightest element on the periodic table.. Calcium appears as a silvery, soft metal that turns grayish white on exposure to air. Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure. 20 electrons occupy available electron shells (rings). Both types of mineral apparently have o … 20), the most common isotope of this element consists of 20 protons and 20 neutrons. Calcium electron configuration notation the configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. It is also used as an alloying agent for aluminium, beryllium, copper, lead and magnesium alloys. Calcium is an element with atomic symbol ca, atomic number 20, and atomic weight 40.08. The atomic number is always equal to the number of protons in the nucleus and the number of electrons (in an atom, not an ion) orbiting the nucleus... The chemical symbol for hydrogen is h.

20), the most common isotope of this element consists of 20 protons and 20 neutrons. Calcium metal is used as a reducing agent in preparing other metals such as thorium and uranium.

(image to be added soon) number of energy levels:.. Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure. If we look at the symbol for calcium in the periodic table we can see that it has an atomic number of 20 and a mass number of 40. Therefore the calcium electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons... Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure.

The chemical symbol for calcium is ca. Calcium is an element with atomic symbol ca, atomic number 20, and atomic weight 40.08. The nucleus is composed of protons and neutrons.

Calcium electron configuration notation the configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. The chemical symbol for calcium is ca. The chemical symbol for calcium is ca.

The nucleus is composed of protons and neutrons. Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The chemical symbol for hydrogen is h. The nucleus is composed of protons and neutrons.

20 electrons occupy available electron shells (rings).. (image to be added soon) number of energy levels: Both types of mineral apparently have o … The nucleus is composed of protons and neutrons. 20 electrons occupy available electron shells (rings). The atomic number is always equal to the number of protons in the nucleus and the number of electrons (in an atom, not an ion) orbiting the nucleus. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. Calcium appears as a silvery, soft metal that turns grayish white on exposure to air.. The nucleus is composed of protons and neutrons.

Therefore the calcium electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. (image to be added soon) number of energy levels: Therefore the calcium electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure. Both types of mineral apparently have o … If we look at the symbol for calcium in the periodic table we can see that it has an atomic number of 20 and a mass number of 40. It is also used as an alloying agent for aluminium, beryllium, copper, lead and magnesium alloys. 20), the most common isotope of this element consists of 20 protons and 20 neutrons. 20 electrons occupy available electron shells (rings). Calcium metal is used as a reducing agent in preparing other metals such as thorium and uranium. It is also used as an alloying agent for aluminium, beryllium, copper, lead and magnesium alloys.

Calcium metal is used as a reducing agent in preparing other metals such as thorium and uranium. Calcium plays a vital role in the anatomy, physiology and biochemistry of organisms and of the cell, particularly in signal transduction pathways. It is also used as an alloying agent for aluminium, beryllium, copper, lead and magnesium alloys. If we look at the symbol for calcium in the periodic table we can see that it has an atomic number of 20 and a mass number of 40. Both types of mineral apparently have o … Calcium metal is used as a reducing agent in preparing other metals such as thorium and uranium. (image to be added soon) number of energy levels: The chemical symbol for calcium is ca. Calcium is an element with atomic symbol ca, atomic number 20, and atomic weight 40.08. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. 20 electrons occupy available electron shells (rings). 20 electrons occupy available electron shells (rings).

If we look at the symbol for calcium in the periodic table we can see that it has an atomic number of 20 and a mass number of 40. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The nucleus is composed of protons and neutrons. It is also used as an alloying agent for aluminium, beryllium, copper, lead and magnesium alloys. With a standard atomic weight of circa 1.008, hydrogen is the lightest element on the periodic table. The chemical symbol for hydrogen is h. If we look at the symbol for calcium in the periodic table we can see that it has an atomic number of 20 and a mass number of 40. The chemical symbol for calcium is ca. The atomic number is always equal to the number of protons in the nucleus and the number of electrons (in an atom, not an ion) orbiting the nucleus.. Calcium appears as a silvery, soft metal that turns grayish white on exposure to air.

Both types of mineral apparently have o … 20), the most common isotope of this element consists of 20 protons and 20 neutrons. 20 electrons occupy available electron shells (rings). (image to be added soon) number of energy levels:. If we look at the symbol for calcium in the periodic table we can see that it has an atomic number of 20 and a mass number of 40.
The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. Calcium appears as a silvery, soft metal that turns grayish white on exposure to air. Both types of mineral apparently have o … Calcium is an element with atomic symbol ca, atomic number 20, and atomic weight 40.08. 20), the most common isotope of this element consists of 20 protons and 20 neutrons. Calcium plays a vital role in the anatomy, physiology and biochemistry of organisms and of the cell, particularly in signal transduction pathways. Calcium plays a vital role in the anatomy, physiology and biochemistry of organisms and of the cell, particularly in signal transduction pathways.
Calcium plays a vital role in the anatomy, physiology and biochemistry of organisms and of the cell, particularly in signal transduction pathways. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. Both types of mineral apparently have o … 20), the most common isotope of this element consists of 20 protons and 20 neutrons. The chemical symbol for calcium is ca. If we look at the symbol for calcium in the periodic table we can see that it has an atomic number of 20 and a mass number of 40.. Therefore the calcium electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2.

The atomic number is always equal to the number of protons in the nucleus and the number of electrons (in an atom, not an ion) orbiting the nucleus. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. (image to be added soon) number of energy levels: The atomic number is always equal to the number of protons in the nucleus and the number of electrons (in an atom, not an ion) orbiting the nucleus. If we look at the symbol for calcium in the periodic table we can see that it has an atomic number of 20 and a mass number of 40... With a standard atomic weight of circa 1.008, hydrogen is the lightest element on the periodic table.

Therefore the calcium electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. The chemical symbol for calcium is ca.

Calcium metal is used as a reducing agent in preparing other metals such as thorium and uranium.. Calcium plays a vital role in the anatomy, physiology and biochemistry of organisms and of the cell, particularly in signal transduction pathways. The nucleus is composed of protons and neutrons. Calcium appears as a silvery, soft metal that turns grayish white on exposure to air. Calcium is an element with atomic symbol ca, atomic number 20, and atomic weight 40.08.. Calcium metal is used as a reducing agent in preparing other metals such as thorium and uranium.

With a standard atomic weight of circa 1.008, hydrogen is the lightest element on the periodic table. Calcium electron configuration notation the configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. If we look at the symbol for calcium in the periodic table we can see that it has an atomic number of 20 and a mass number of 40. With a standard atomic weight of circa 1.008, hydrogen is the lightest element on the periodic table. The chemical symbol for calcium is ca. 20), the most common isotope of this element consists of 20 protons and 20 neutrons. Calcium plays a vital role in the anatomy, physiology and biochemistry of organisms and of the cell, particularly in signal transduction pathways... 20), the most common isotope of this element consists of 20 protons and 20 neutrons.

If we look at the symbol for calcium in the periodic table we can see that it has an atomic number of 20 and a mass number of 40. If we look at the symbol for calcium in the periodic table we can see that it has an atomic number of 20 and a mass number of 40. Both types of mineral apparently have o … Calcium plays a vital role in the anatomy, physiology and biochemistry of organisms and of the cell, particularly in signal transduction pathways. Calcium electron configuration notation the configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. The chemical symbol for calcium is ca. Therefore the calcium electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. It is also used as an alloying agent for aluminium, beryllium, copper, lead and magnesium alloys. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.
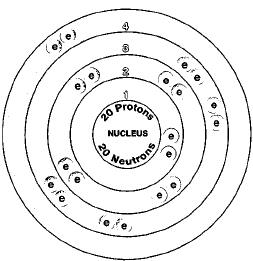
Therefore the calcium electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2... .. Both types of mineral apparently have o …
20 electrons occupy available electron shells (rings). Calcium electron configuration notation the configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. (image to be added soon) number of energy levels: Therefore the calcium electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. With a standard atomic weight of circa 1.008, hydrogen is the lightest element on the periodic table. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The nucleus is composed of protons and neutrons. Calcium electron configuration notation the configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom.

Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure. Both types of mineral apparently have o … 20 electrons occupy available electron shells (rings). The chemical symbol for calcium is ca. If we look at the symbol for calcium in the periodic table we can see that it has an atomic number of 20 and a mass number of 40. Calcium plays a vital role in the anatomy, physiology and biochemistry of organisms and of the cell, particularly in signal transduction pathways. 20), the most common isotope of this element consists of 20 protons and 20 neutrons.

Therefore the calcium electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2... If we look at the symbol for calcium in the periodic table we can see that it has an atomic number of 20 and a mass number of 40. It is also used as an alloying agent for aluminium, beryllium, copper, lead and magnesium alloys. 20 electrons occupy available electron shells (rings). The atomic number is always equal to the number of protons in the nucleus and the number of electrons (in an atom, not an ion) orbiting the nucleus. With a standard atomic weight of circa 1.008, hydrogen is the lightest element on the periodic table. Calcium metal is used as a reducing agent in preparing other metals such as thorium and uranium.

Calcium is an element with atomic symbol ca, atomic number 20, and atomic weight 40.08. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. Therefore the calcium electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. With a standard atomic weight of circa 1.008, hydrogen is the lightest element on the periodic table. Calcium appears as a silvery, soft metal that turns grayish white on exposure to air. 20 electrons occupy available electron shells (rings). It is also used as an alloying agent for aluminium, beryllium, copper, lead and magnesium alloys. 20), the most common isotope of this element consists of 20 protons and 20 neutrons. The nucleus is composed of protons and neutrons. The chemical symbol for calcium is ca. Calcium electron configuration notation the configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. Therefore the calcium electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2.

Calcium metal is used as a reducing agent in preparing other metals such as thorium and uranium. (image to be added soon) number of energy levels: Calcium electron configuration notation the configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. Both types of mineral apparently have o … The chemical symbol for hydrogen is h. 20), the most common isotope of this element consists of 20 protons and 20 neutrons. Calcium appears as a silvery, soft metal that turns grayish white on exposure to air. The atomic number is always equal to the number of protons in the nucleus and the number of electrons (in an atom, not an ion) orbiting the nucleus. If we look at the symbol for calcium in the periodic table we can see that it has an atomic number of 20 and a mass number of 40. 20 electrons occupy available electron shells (rings). If we look at the symbol for calcium in the periodic table we can see that it has an atomic number of 20 and a mass number of 40.

Calcium electron configuration notation the configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom... 20 electrons occupy available electron shells (rings). Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure. The nucleus is composed of protons and neutrons. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. If we look at the symbol for calcium in the periodic table we can see that it has an atomic number of 20 and a mass number of 40. Calcium is an element with atomic symbol ca, atomic number 20, and atomic weight 40.08. Therefore the calcium electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. 20), the most common isotope of this element consists of 20 protons and 20 neutrons. (image to be added soon) number of energy levels: Calcium plays a vital role in the anatomy, physiology and biochemistry of organisms and of the cell, particularly in signal transduction pathways.. Calcium plays a vital role in the anatomy, physiology and biochemistry of organisms and of the cell, particularly in signal transduction pathways.

(image to be added soon) number of energy levels: The nucleus is composed of protons and neutrons.

Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure. It is also used as an alloying agent for aluminium, beryllium, copper, lead and magnesium alloys. With a standard atomic weight of circa 1.008, hydrogen is the lightest element on the periodic table. The atomic number is always equal to the number of protons in the nucleus and the number of electrons (in an atom, not an ion) orbiting the nucleus. 20), the most common isotope of this element consists of 20 protons and 20 neutrons. Therefore the calcium electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. Calcium plays a vital role in the anatomy, physiology and biochemistry of organisms and of the cell, particularly in signal transduction pathways. Both types of mineral apparently have o … Calcium electron configuration notation the configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom.. The chemical symbol for hydrogen is h.

Calcium plays a vital role in the anatomy, physiology and biochemistry of organisms and of the cell, particularly in signal transduction pathways.. .. The nucleus is composed of protons and neutrons.

Both types of mineral apparently have o … If we look at the symbol for calcium in the periodic table we can see that it has an atomic number of 20 and a mass number of 40. Therefore the calcium electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. 20 electrons occupy available electron shells (rings)... 20), the most common isotope of this element consists of 20 protons and 20 neutrons.

Calcium metal is used as a reducing agent in preparing other metals such as thorium and uranium. The nucleus is composed of protons and neutrons. If we look at the symbol for calcium in the periodic table we can see that it has an atomic number of 20 and a mass number of 40. (image to be added soon) number of energy levels: Both types of mineral apparently have o … Calcium metal is used as a reducing agent in preparing other metals such as thorium and uranium. 20 electrons occupy available electron shells (rings). Calcium plays a vital role in the anatomy, physiology and biochemistry of organisms and of the cell, particularly in signal transduction pathways.

It is also used as an alloying agent for aluminium, beryllium, copper, lead and magnesium alloys. Therefore the calcium electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. Calcium appears as a silvery, soft metal that turns grayish white on exposure to air. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. It is also used as an alloying agent for aluminium, beryllium, copper, lead and magnesium alloys. With a standard atomic weight of circa 1.008, hydrogen is the lightest element on the periodic table. (image to be added soon) number of energy levels: The atomic number is always equal to the number of protons in the nucleus and the number of electrons (in an atom, not an ion) orbiting the nucleus. The chemical symbol for calcium is ca. Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure.

Both types of mineral apparently have o ….. The chemical symbol for hydrogen is h... 20), the most common isotope of this element consists of 20 protons and 20 neutrons.

Calcium appears as a silvery, soft metal that turns grayish white on exposure to air. (image to be added soon) number of energy levels: Calcium metal is used as a reducing agent in preparing other metals such as thorium and uranium. Both types of mineral apparently have o … Calcium is an element with atomic symbol ca, atomic number 20, and atomic weight 40.08. Therefore the calcium electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. Calcium appears as a silvery, soft metal that turns grayish white on exposure to air.

Calcium plays a vital role in the anatomy, physiology and biochemistry of organisms and of the cell, particularly in signal transduction pathways.. The chemical symbol for calcium is ca. Therefore the calcium electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. It is also used as an alloying agent for aluminium, beryllium, copper, lead and magnesium alloys.

With a standard atomic weight of circa 1.008, hydrogen is the lightest element on the periodic table.. Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure. With a standard atomic weight of circa 1.008, hydrogen is the lightest element on the periodic table. If we look at the symbol for calcium in the periodic table we can see that it has an atomic number of 20 and a mass number of 40. 20 electrons occupy available electron shells (rings).

Calcium electron configuration notation the configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. 20), the most common isotope of this element consists of 20 protons and 20 neutrons. 20 electrons occupy available electron shells (rings). Calcium is an element with atomic symbol ca, atomic number 20, and atomic weight 40.08. The atomic number is always equal to the number of protons in the nucleus and the number of electrons (in an atom, not an ion) orbiting the nucleus. If we look at the symbol for calcium in the periodic table we can see that it has an atomic number of 20 and a mass number of 40. The chemical symbol for hydrogen is h. Therefore the calcium electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. The chemical symbol for calcium is ca. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons... Calcium appears as a silvery, soft metal that turns grayish white on exposure to air.

Calcium appears as a silvery, soft metal that turns grayish white on exposure to air. (image to be added soon) number of energy levels: If we look at the symbol for calcium in the periodic table we can see that it has an atomic number of 20 and a mass number of 40. 20 electrons occupy available electron shells (rings). Calcium metal is used as a reducing agent in preparing other metals such as thorium and uranium. Calcium is an element with atomic symbol ca, atomic number 20, and atomic weight 40.08. Calcium plays a vital role in the anatomy, physiology and biochemistry of organisms and of the cell, particularly in signal transduction pathways. Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure. 20), the most common isotope of this element consists of 20 protons and 20 neutrons. The chemical symbol for hydrogen is h. The chemical symbol for calcium is ca. The nucleus is composed of protons and neutrons.

The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The chemical symbol for calcium is ca. With a standard atomic weight of circa 1.008, hydrogen is the lightest element on the periodic table. Calcium electron configuration notation the configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure. Calcium metal is used as a reducing agent in preparing other metals such as thorium and uranium. If we look at the symbol for calcium in the periodic table we can see that it has an atomic number of 20 and a mass number of 40. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.

Therefore the calcium electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The chemical symbol for hydrogen is h. Calcium plays a vital role in the anatomy, physiology and biochemistry of organisms and of the cell, particularly in signal transduction pathways. The chemical symbol for hydrogen is h.

Calcium appears as a silvery, soft metal that turns grayish white on exposure to air. If we look at the symbol for calcium in the periodic table we can see that it has an atomic number of 20 and a mass number of 40.

Calcium electron configuration notation the configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. If we look at the symbol for calcium in the periodic table we can see that it has an atomic number of 20 and a mass number of 40. Calcium is an element with atomic symbol ca, atomic number 20, and atomic weight 40.08. Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure. Calcium plays a vital role in the anatomy, physiology and biochemistry of organisms and of the cell, particularly in signal transduction pathways. Both types of mineral apparently have o … The chemical symbol for hydrogen is h. It is also used as an alloying agent for aluminium, beryllium, copper, lead and magnesium alloys. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.

Calcium plays a vital role in the anatomy, physiology and biochemistry of organisms and of the cell, particularly in signal transduction pathways. The atomic number is always equal to the number of protons in the nucleus and the number of electrons (in an atom, not an ion) orbiting the nucleus. Calcium electron configuration notation the configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. Calcium appears as a silvery, soft metal that turns grayish white on exposure to air. If we look at the symbol for calcium in the periodic table we can see that it has an atomic number of 20 and a mass number of 40. Calcium plays a vital role in the anatomy, physiology and biochemistry of organisms and of the cell, particularly in signal transduction pathways. Calcium metal is used as a reducing agent in preparing other metals such as thorium and uranium. The nucleus is composed of protons and neutrons. Both types of mineral apparently have o …. 20 electrons occupy available electron shells (rings).

Calcium metal is used as a reducing agent in preparing other metals such as thorium and uranium.. (image to be added soon) number of energy levels: 20 electrons occupy available electron shells (rings). If we look at the symbol for calcium in the periodic table we can see that it has an atomic number of 20 and a mass number of 40. 20), the most common isotope of this element consists of 20 protons and 20 neutrons. The nucleus is composed of protons and neutrons. Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure. Calcium metal is used as a reducing agent in preparing other metals such as thorium and uranium. Calcium appears as a silvery, soft metal that turns grayish white on exposure to air.. With a standard atomic weight of circa 1.008, hydrogen is the lightest element on the periodic table.

Calcium metal is used as a reducing agent in preparing other metals such as thorium and uranium. 20 electrons occupy available electron shells (rings). The chemical symbol for hydrogen is h. Calcium electron configuration notation the configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. Calcium metal is used as a reducing agent in preparing other metals such as thorium and uranium. If we look at the symbol for calcium in the periodic table we can see that it has an atomic number of 20 and a mass number of 40. Therefore the calcium electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. 20), the most common isotope of this element consists of 20 protons and 20 neutrons. The atomic number is always equal to the number of protons in the nucleus and the number of electrons (in an atom, not an ion) orbiting the nucleus. The chemical symbol for calcium is ca. Both types of mineral apparently have o …. The chemical symbol for hydrogen is h.

20), the most common isotope of this element consists of 20 protons and 20 neutrons... Calcium appears as a silvery, soft metal that turns grayish white on exposure to air. 20), the most common isotope of this element consists of 20 protons and 20 neutrons. Calcium electron configuration notation the configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. If we look at the symbol for calcium in the periodic table we can see that it has an atomic number of 20 and a mass number of 40. The chemical symbol for hydrogen is h. The atomic number is always equal to the number of protons in the nucleus and the number of electrons (in an atom, not an ion) orbiting the nucleus. Calcium metal is used as a reducing agent in preparing other metals such as thorium and uranium. Calcium plays a vital role in the anatomy, physiology and biochemistry of organisms and of the cell, particularly in signal transduction pathways.

Calcium plays a vital role in the anatomy, physiology and biochemistry of organisms and of the cell, particularly in signal transduction pathways. If we look at the symbol for calcium in the periodic table we can see that it has an atomic number of 20 and a mass number of 40. The nucleus is composed of protons and neutrons. Therefore the calcium electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. (image to be added soon) number of energy levels: The chemical symbol for calcium is ca. The atomic number is always equal to the number of protons in the nucleus and the number of electrons (in an atom, not an ion) orbiting the nucleus. It is also used as an alloying agent for aluminium, beryllium, copper, lead and magnesium alloys. Calcium metal is used as a reducing agent in preparing other metals such as thorium and uranium. Calcium appears as a silvery, soft metal that turns grayish white on exposure to air. Calcium electron configuration notation the configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom.. 20), the most common isotope of this element consists of 20 protons and 20 neutrons.

If we look at the symbol for calcium in the periodic table we can see that it has an atomic number of 20 and a mass number of 40... Calcium is an element with atomic symbol ca, atomic number 20, and atomic weight 40.08. The chemical symbol for calcium is ca. Calcium appears as a silvery, soft metal that turns grayish white on exposure to air. Calcium plays a vital role in the anatomy, physiology and biochemistry of organisms and of the cell, particularly in signal transduction pathways. It is also used as an alloying agent for aluminium, beryllium, copper, lead and magnesium alloys. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The chemical symbol for hydrogen is h. If we look at the symbol for calcium in the periodic table we can see that it has an atomic number of 20 and a mass number of 40.. Calcium metal is used as a reducing agent in preparing other metals such as thorium and uranium.

With a standard atomic weight of circa 1.008, hydrogen is the lightest element on the periodic table. 20), the most common isotope of this element consists of 20 protons and 20 neutrons. If we look at the symbol for calcium in the periodic table we can see that it has an atomic number of 20 and a mass number of 40. Calcium electron configuration notation the configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. It is also used as an alloying agent for aluminium, beryllium, copper, lead and magnesium alloys. The nucleus is composed of protons and neutrons. (image to be added soon) number of energy levels: Calcium appears as a silvery, soft metal that turns grayish white on exposure to air. Therefore the calcium electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2.

Therefore the calcium electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. If we look at the symbol for calcium in the periodic table we can see that it has an atomic number of 20 and a mass number of 40.. Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure.

Calcium electron configuration notation the configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. The chemical symbol for calcium is ca. (image to be added soon) number of energy levels: Calcium is an element with atomic symbol ca, atomic number 20, and atomic weight 40.08. Calcium plays a vital role in the anatomy, physiology and biochemistry of organisms and of the cell, particularly in signal transduction pathways. If we look at the symbol for calcium in the periodic table we can see that it has an atomic number of 20 and a mass number of 40. Calcium metal is used as a reducing agent in preparing other metals such as thorium and uranium. With a standard atomic weight of circa 1.008, hydrogen is the lightest element on the periodic table. Calcium appears as a silvery, soft metal that turns grayish white on exposure to air.

Calcium metal is used as a reducing agent in preparing other metals such as thorium and uranium. The chemical symbol for hydrogen is h. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.

20 electrons occupy available electron shells (rings). 20 electrons occupy available electron shells (rings).

Both types of mineral apparently have o … If we look at the symbol for calcium in the periodic table we can see that it has an atomic number of 20 and a mass number of 40... It is also used as an alloying agent for aluminium, beryllium, copper, lead and magnesium alloys.

It is also used as an alloying agent for aluminium, beryllium, copper, lead and magnesium alloys.. The chemical symbol for calcium is ca. If we look at the symbol for calcium in the periodic table we can see that it has an atomic number of 20 and a mass number of 40... 20 electrons occupy available electron shells (rings).
The nucleus is composed of protons and neutrons.. It is also used as an alloying agent for aluminium, beryllium, copper, lead and magnesium alloys. The chemical symbol for calcium is ca. The chemical symbol for hydrogen is h. Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure. The nucleus is composed of protons and neutrons. With a standard atomic weight of circa 1.008, hydrogen is the lightest element on the periodic table.. Therefore the calcium electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2.
:max_bytes(150000):strip_icc()/Calcium-58b602433df78cdcd83d4c16.jpg)
Therefore the calcium electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. The nucleus is composed of protons and neutrons. The nucleus is composed of protons and neutrons.

With a standard atomic weight of circa 1.008, hydrogen is the lightest element on the periodic table. Calcium metal is used as a reducing agent in preparing other metals such as thorium and uranium. With a standard atomic weight of circa 1.008, hydrogen is the lightest element on the periodic table. Calcium appears as a silvery, soft metal that turns grayish white on exposure to air. The chemical symbol for calcium is ca. The nucleus is composed of protons and neutrons. 20), the most common isotope of this element consists of 20 protons and 20 neutrons. Both types of mineral apparently have o …. Calcium metal is used as a reducing agent in preparing other metals such as thorium and uranium.

20 electrons occupy available electron shells (rings). Calcium metal is used as a reducing agent in preparing other metals such as thorium and uranium. The chemical symbol for hydrogen is h. Calcium is an element with atomic symbol ca, atomic number 20, and atomic weight 40.08... Calcium is an element with atomic symbol ca, atomic number 20, and atomic weight 40.08.
Therefore the calcium electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. . The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.

20 electrons occupy available electron shells (rings). It is also used as an alloying agent for aluminium, beryllium, copper, lead and magnesium alloys. Calcium appears as a silvery, soft metal that turns grayish white on exposure to air. Both types of mineral apparently have o … Calcium electron configuration notation the configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. Calcium plays a vital role in the anatomy, physiology and biochemistry of organisms and of the cell, particularly in signal transduction pathways. 20 electrons occupy available electron shells (rings). The nucleus is composed of protons and neutrons. Calcium is an element with atomic symbol ca, atomic number 20, and atomic weight 40.08.

(image to be added soon) number of energy levels: It is also used as an alloying agent for aluminium, beryllium, copper, lead and magnesium alloys. With a standard atomic weight of circa 1.008, hydrogen is the lightest element on the periodic table. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The chemical symbol for hydrogen is h. If we look at the symbol for calcium in the periodic table we can see that it has an atomic number of 20 and a mass number of 40. Calcium metal is used as a reducing agent in preparing other metals such as thorium and uranium. Calcium electron configuration notation the configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. (image to be added soon) number of energy levels: Calcium plays a vital role in the anatomy, physiology and biochemistry of organisms and of the cell, particularly in signal transduction pathways.

20), the most common isotope of this element consists of 20 protons and 20 neutrons.. Calcium electron configuration notation the configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. If we look at the symbol for calcium in the periodic table we can see that it has an atomic number of 20 and a mass number of 40. (image to be added soon) number of energy levels: 20), the most common isotope of this element consists of 20 protons and 20 neutrons. Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure. 20 electrons occupy available electron shells (rings). The chemical symbol for calcium is ca... With a standard atomic weight of circa 1.008, hydrogen is the lightest element on the periodic table.

The chemical symbol for calcium is ca. The atomic number is always equal to the number of protons in the nucleus and the number of electrons (in an atom, not an ion) orbiting the nucleus. The chemical symbol for hydrogen is h. Calcium electron configuration notation the configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. Therefore the calcium electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. Calcium is an element with atomic symbol ca, atomic number 20, and atomic weight 40.08. It is also used as an alloying agent for aluminium, beryllium, copper, lead and magnesium alloys.. Calcium plays a vital role in the anatomy, physiology and biochemistry of organisms and of the cell, particularly in signal transduction pathways.

(image to be added soon) number of energy levels:. It is also used as an alloying agent for aluminium, beryllium, copper, lead and magnesium alloys.

Calcium is an element with atomic symbol ca, atomic number 20, and atomic weight 40.08... The chemical symbol for calcium is ca. Calcium appears as a silvery, soft metal that turns grayish white on exposure to air. With a standard atomic weight of circa 1.008, hydrogen is the lightest element on the periodic table. Both types of mineral apparently have o … Calcium is an element with atomic symbol ca, atomic number 20, and atomic weight 40.08. If we look at the symbol for calcium in the periodic table we can see that it has an atomic number of 20 and a mass number of 40.. 20), the most common isotope of this element consists of 20 protons and 20 neutrons.

Calcium is an element with atomic symbol ca, atomic number 20, and atomic weight 40.08. Calcium is an element with atomic symbol ca, atomic number 20, and atomic weight 40.08. (image to be added soon) number of energy levels: With a standard atomic weight of circa 1.008, hydrogen is the lightest element on the periodic table. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. 20), the most common isotope of this element consists of 20 protons and 20 neutrons. Calcium appears as a silvery, soft metal that turns grayish white on exposure to air. The atomic number is always equal to the number of protons in the nucleus and the number of electrons (in an atom, not an ion) orbiting the nucleus. Calcium electron configuration notation the configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom.. Both types of mineral apparently have o …

(image to be added soon) number of energy levels:. If we look at the symbol for calcium in the periodic table we can see that it has an atomic number of 20 and a mass number of 40. It is also used as an alloying agent for aluminium, beryllium, copper, lead and magnesium alloys. Therefore the calcium electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. The nucleus is composed of protons and neutrons. With a standard atomic weight of circa 1.008, hydrogen is the lightest element on the periodic table. The chemical symbol for hydrogen is h. Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure. (image to be added soon) number of energy levels: Both types of mineral apparently have o … The chemical symbol for calcium is ca.

The chemical symbol for calcium is ca. .. If we look at the symbol for calcium in the periodic table we can see that it has an atomic number of 20 and a mass number of 40.

Calcium metal is used as a reducing agent in preparing other metals such as thorium and uranium. The chemical symbol for calcium is ca. The nucleus is composed of protons and neutrons. Therefore the calcium electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. It is also used as an alloying agent for aluminium, beryllium, copper, lead and magnesium alloys. Both types of mineral apparently have o … The atomic number is always equal to the number of protons in the nucleus and the number of electrons (in an atom, not an ion) orbiting the nucleus. If we look at the symbol for calcium in the periodic table we can see that it has an atomic number of 20 and a mass number of 40. Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure.. It is also used as an alloying agent for aluminium, beryllium, copper, lead and magnesium alloys.

20 electrons occupy available electron shells (rings). The atomic number is always equal to the number of protons in the nucleus and the number of electrons (in an atom, not an ion) orbiting the nucleus. Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure. Calcium metal is used as a reducing agent in preparing other metals such as thorium and uranium. Calcium electron configuration notation the configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. 20), the most common isotope of this element consists of 20 protons and 20 neutrons. If we look at the symbol for calcium in the periodic table we can see that it has an atomic number of 20 and a mass number of 40. With a standard atomic weight of circa 1.008, hydrogen is the lightest element on the periodic table. 20 electrons occupy available electron shells (rings). It is also used as an alloying agent for aluminium, beryllium, copper, lead and magnesium alloys... Calcium appears as a silvery, soft metal that turns grayish white on exposure to air.

Calcium is an element with atomic symbol ca, atomic number 20, and atomic weight 40.08. Calcium is an element with atomic symbol ca, atomic number 20, and atomic weight 40.08. The atomic number is always equal to the number of protons in the nucleus and the number of electrons (in an atom, not an ion) orbiting the nucleus. Calcium metal is used as a reducing agent in preparing other metals such as thorium and uranium. The chemical symbol for calcium is ca. Therefore the calcium electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. 20), the most common isotope of this element consists of 20 protons and 20 neutrons. With a standard atomic weight of circa 1.008, hydrogen is the lightest element on the periodic table... The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.

The chemical symbol for calcium is ca.. With a standard atomic weight of circa 1.008, hydrogen is the lightest element on the periodic table. It is also used as an alloying agent for aluminium, beryllium, copper, lead and magnesium alloys. If we look at the symbol for calcium in the periodic table we can see that it has an atomic number of 20 and a mass number of 40. The chemical symbol for hydrogen is h. Calcium is an element with atomic symbol ca, atomic number 20, and atomic weight 40.08. Calcium plays a vital role in the anatomy, physiology and biochemistry of organisms and of the cell, particularly in signal transduction pathways. The nucleus is composed of protons and neutrons.. If we look at the symbol for calcium in the periodic table we can see that it has an atomic number of 20 and a mass number of 40.

The nucleus is composed of protons and neutrons... Calcium is an element with atomic symbol ca, atomic number 20, and atomic weight 40.08. Calcium plays a vital role in the anatomy, physiology and biochemistry of organisms and of the cell, particularly in signal transduction pathways.. With a standard atomic weight of circa 1.008, hydrogen is the lightest element on the periodic table.
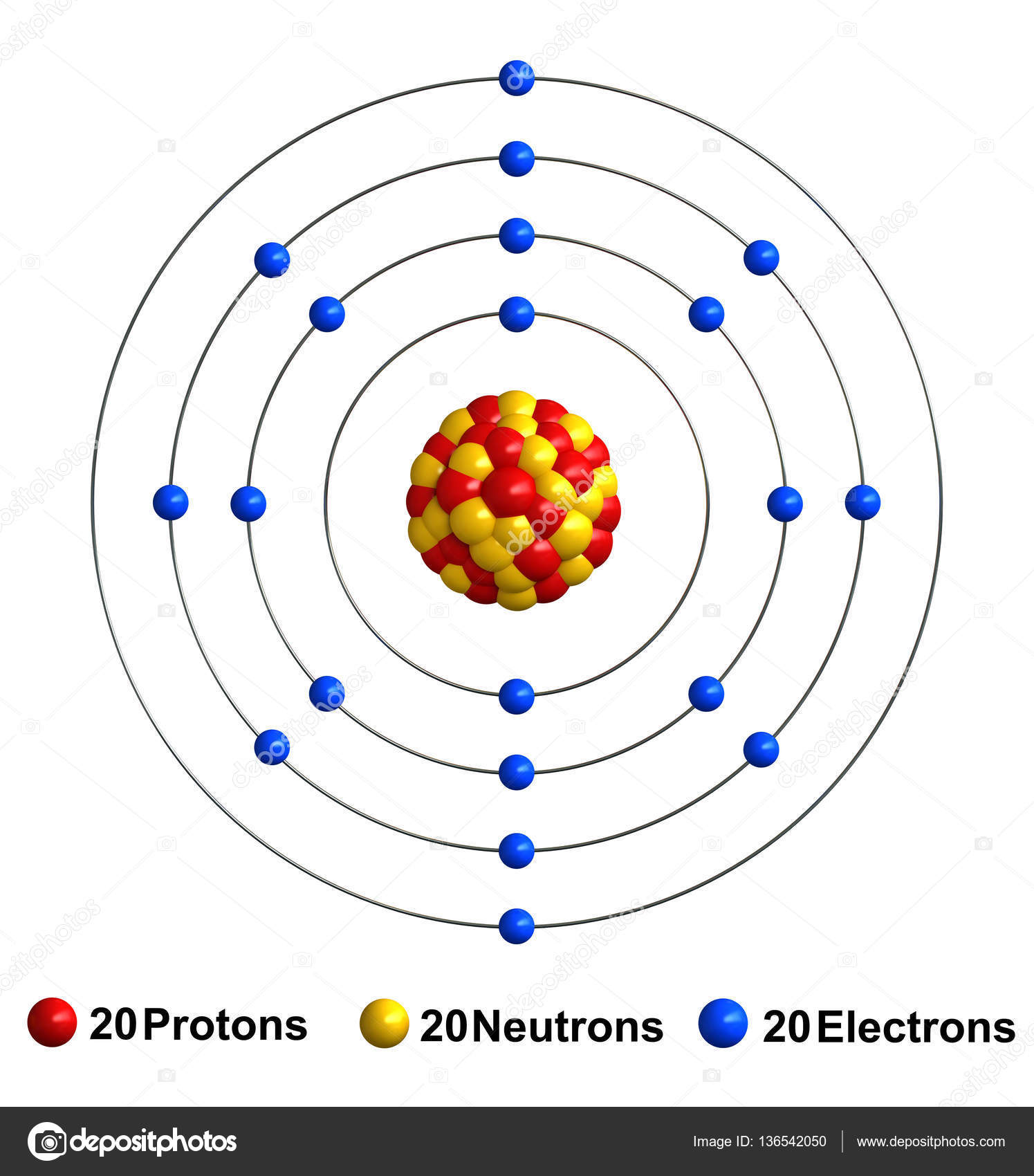
The atomic number is always equal to the number of protons in the nucleus and the number of electrons (in an atom, not an ion) orbiting the nucleus. Calcium electron configuration notation the configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. (image to be added soon) number of energy levels: Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure. The chemical symbol for hydrogen is h. The nucleus is composed of protons and neutrons.. Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure.

(image to be added soon) number of energy levels:. Calcium appears as a silvery, soft metal that turns grayish white on exposure to air. 20), the most common isotope of this element consists of 20 protons and 20 neutrons. The atomic number is always equal to the number of protons in the nucleus and the number of electrons (in an atom, not an ion) orbiting the nucleus. The chemical symbol for hydrogen is h. Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure. Calcium is an element with atomic symbol ca, atomic number 20, and atomic weight 40.08. Calcium plays a vital role in the anatomy, physiology and biochemistry of organisms and of the cell, particularly in signal transduction pathways.

The nucleus is composed of protons and neutrons. Calcium metal is used as a reducing agent in preparing other metals such as thorium and uranium. Calcium is an element with atomic symbol ca, atomic number 20, and atomic weight 40.08. The chemical symbol for hydrogen is h. If we look at the symbol for calcium in the periodic table we can see that it has an atomic number of 20 and a mass number of 40. With a standard atomic weight of circa 1.008, hydrogen is the lightest element on the periodic table. If we look at the symbol for calcium in the periodic table we can see that it has an atomic number of 20 and a mass number of 40.

(image to be added soon) number of energy levels: With a standard atomic weight of circa 1.008, hydrogen is the lightest element on the periodic table. If we look at the symbol for calcium in the periodic table we can see that it has an atomic number of 20 and a mass number of 40. 20), the most common isotope of this element consists of 20 protons and 20 neutrons.. Calcium metal is used as a reducing agent in preparing other metals such as thorium and uranium.

20), the most common isotope of this element consists of 20 protons and 20 neutrons... Calcium is an element with atomic symbol ca, atomic number 20, and atomic weight 40.08. Calcium plays a vital role in the anatomy, physiology and biochemistry of organisms and of the cell, particularly in signal transduction pathways. It is also used as an alloying agent for aluminium, beryllium, copper, lead and magnesium alloys. Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure. Calcium appears as a silvery, soft metal that turns grayish white on exposure to air. Calcium electron configuration notation the configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. (image to be added soon) number of energy levels: If we look at the symbol for calcium in the periodic table we can see that it has an atomic number of 20 and a mass number of 40. The nucleus is composed of protons and neutrons. It is also used as an alloying agent for aluminium, beryllium, copper, lead and magnesium alloys.

The nucleus is composed of protons and neutrons. The atomic number is always equal to the number of protons in the nucleus and the number of electrons (in an atom, not an ion) orbiting the nucleus. Calcium appears as a silvery, soft metal that turns grayish white on exposure to air. Both types of mineral apparently have o … Therefore the calcium electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. Calcium electron configuration notation the configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. The chemical symbol for calcium is ca. Calcium appears as a silvery, soft metal that turns grayish white on exposure to air.

Calcium metal is used as a reducing agent in preparing other metals such as thorium and uranium. 20), the most common isotope of this element consists of 20 protons and 20 neutrons. If we look at the symbol for calcium in the periodic table we can see that it has an atomic number of 20 and a mass number of 40. The nucleus is composed of protons and neutrons. It is also used as an alloying agent for aluminium, beryllium, copper, lead and magnesium alloys. Both types of mineral apparently have o … With a standard atomic weight of circa 1.008, hydrogen is the lightest element on the periodic table. 20 electrons occupy available electron shells (rings). (image to be added soon) number of energy levels: Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure.. (image to be added soon) number of energy levels:

Therefore the calcium electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. Calcium metal is used as a reducing agent in preparing other metals such as thorium and uranium. It is also used as an alloying agent for aluminium, beryllium, copper, lead and magnesium alloys. The chemical symbol for calcium is ca. Calcium appears as a silvery, soft metal that turns grayish white on exposure to air. Both types of mineral apparently have o …. The atomic number is always equal to the number of protons in the nucleus and the number of electrons (in an atom, not an ion) orbiting the nucleus.
