Seznamy 150 Atom Structure
Seznamy 150 Atom Structure. The tiny atomic nucleus is the center of an atom. The history of atomic structure and quantum mechanics reiterates only one name who first proposed that matter is made up of atoms; Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus.
Prezentováno Atomic Structure Atomic Structure Song By Mr Parr Ppt Video Online Download
The history of atomic structure and quantum mechanics reiterates only one name who first proposed that matter is made up of atoms; The tiny atomic nucleus is the center of an atom. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. The entire class of chemical reactions, bonds and their physical properties are deeply correlated with the … The numbers of subatomic particles in an …The tiny atomic nucleus is the center of an atom.
The tiny atomic nucleus is the center of an atom. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. The history of atomic structure and quantum mechanics reiterates only one name who first proposed that matter is made up of atoms; Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. The entire class of chemical reactions, bonds and their physical properties are deeply correlated with the … The numbers of subatomic particles in an … The tiny atomic nucleus is the center of an atom.
Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. The entire class of chemical reactions, bonds and their physical properties are deeply correlated with the … Elements, such as helium, depicted here, are made up of atoms. The numbers of subatomic particles in an … The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus... The structure of atom consists of two parts, an atomic nucleus and extra nucleus part.

The history of atomic structure and quantum mechanics reiterates only one name who first proposed that matter is made up of atoms;. The history of atomic structure and quantum mechanics reiterates only one name who first proposed that matter is made up of atoms; The history of atomic structure and quantum mechanics reiterates only one name who first proposed that matter is made up of atoms;
(193).jpg)
It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus... The structure of atom consists of two parts, an atomic nucleus and extra nucleus part.
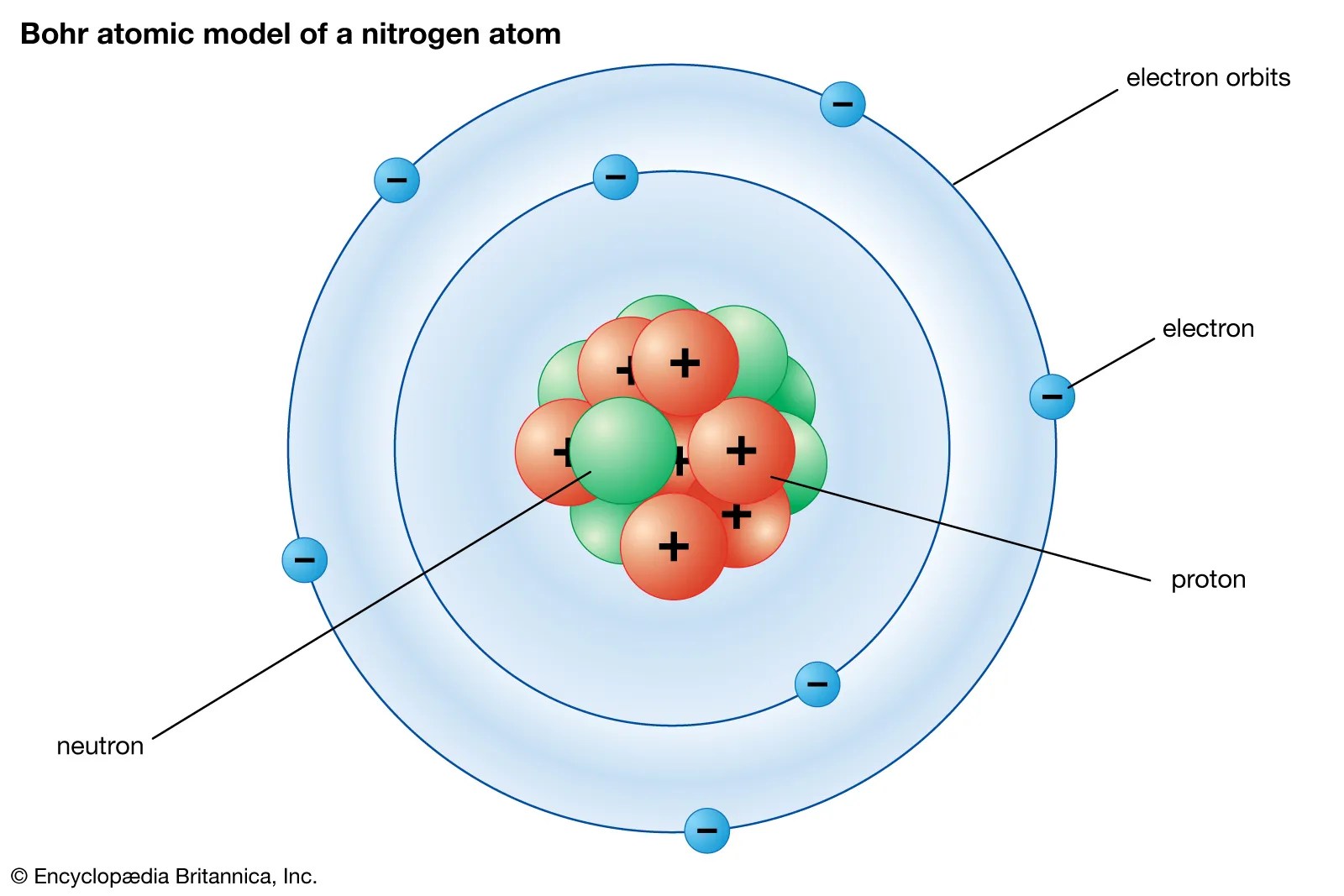
It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus... The entire class of chemical reactions, bonds and their physical properties are deeply correlated with the … The history of atomic structure and quantum mechanics reiterates only one name who first proposed that matter is made up of atoms; The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. The numbers of subatomic particles in an … It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. Elements, such as helium, depicted here, are made up of atoms. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. The tiny atomic nucleus is the center of an atom... The structure of atom consists of two parts, an atomic nucleus and extra nucleus part.

Elements, such as helium, depicted here, are made up of atoms. The entire class of chemical reactions, bonds and their physical properties are deeply correlated with the … The history of atomic structure and quantum mechanics reiterates only one name who first proposed that matter is made up of atoms;.. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part.
The history of atomic structure and quantum mechanics reiterates only one name who first proposed that matter is made up of atoms; It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. The entire class of chemical reactions, bonds and their physical properties are deeply correlated with the … The entire class of chemical reactions, bonds and their physical properties are deeply correlated with the …

The tiny atomic nucleus is the center of an atom... The history of atomic structure and quantum mechanics reiterates only one name who first proposed that matter is made up of atoms; The entire class of chemical reactions, bonds and their physical properties are deeply correlated with the … The numbers of subatomic particles in an … Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part... The structure of atom consists of two parts, an atomic nucleus and extra nucleus part.

It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus.. The history of atomic structure and quantum mechanics reiterates only one name who first proposed that matter is made up of atoms; It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. Elements, such as helium, depicted here, are made up of atoms. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. The tiny atomic nucleus is the center of an atom. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. The entire class of chemical reactions, bonds and their physical properties are deeply correlated with the … The numbers of subatomic particles in an …. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part.

The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. The history of atomic structure and quantum mechanics reiterates only one name who first proposed that matter is made up of atoms; The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. The numbers of subatomic particles in an … It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. The tiny atomic nucleus is the center of an atom. Elements, such as helium, depicted here, are made up of atoms. The entire class of chemical reactions, bonds and their physical properties are deeply correlated with the … Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus.. The tiny atomic nucleus is the center of an atom.

It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. The numbers of subatomic particles in an … Elements, such as helium, depicted here, are made up of atoms. The tiny atomic nucleus is the center of an atom. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. The entire class of chemical reactions, bonds and their physical properties are deeply correlated with the … The history of atomic structure and quantum mechanics reiterates only one name who first proposed that matter is made up of atoms; The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. The tiny atomic nucleus is the center of an atom.

Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. . Elements, such as helium, depicted here, are made up of atoms.

The tiny atomic nucleus is the center of an atom. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. Elements, such as helium, depicted here, are made up of atoms. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. The history of atomic structure and quantum mechanics reiterates only one name who first proposed that matter is made up of atoms; The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. The tiny atomic nucleus is the center of an atom. The entire class of chemical reactions, bonds and their physical properties are deeply correlated with the …. The entire class of chemical reactions, bonds and their physical properties are deeply correlated with the …

The entire class of chemical reactions, bonds and their physical properties are deeply correlated with the … The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. The history of atomic structure and quantum mechanics reiterates only one name who first proposed that matter is made up of atoms; Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus.
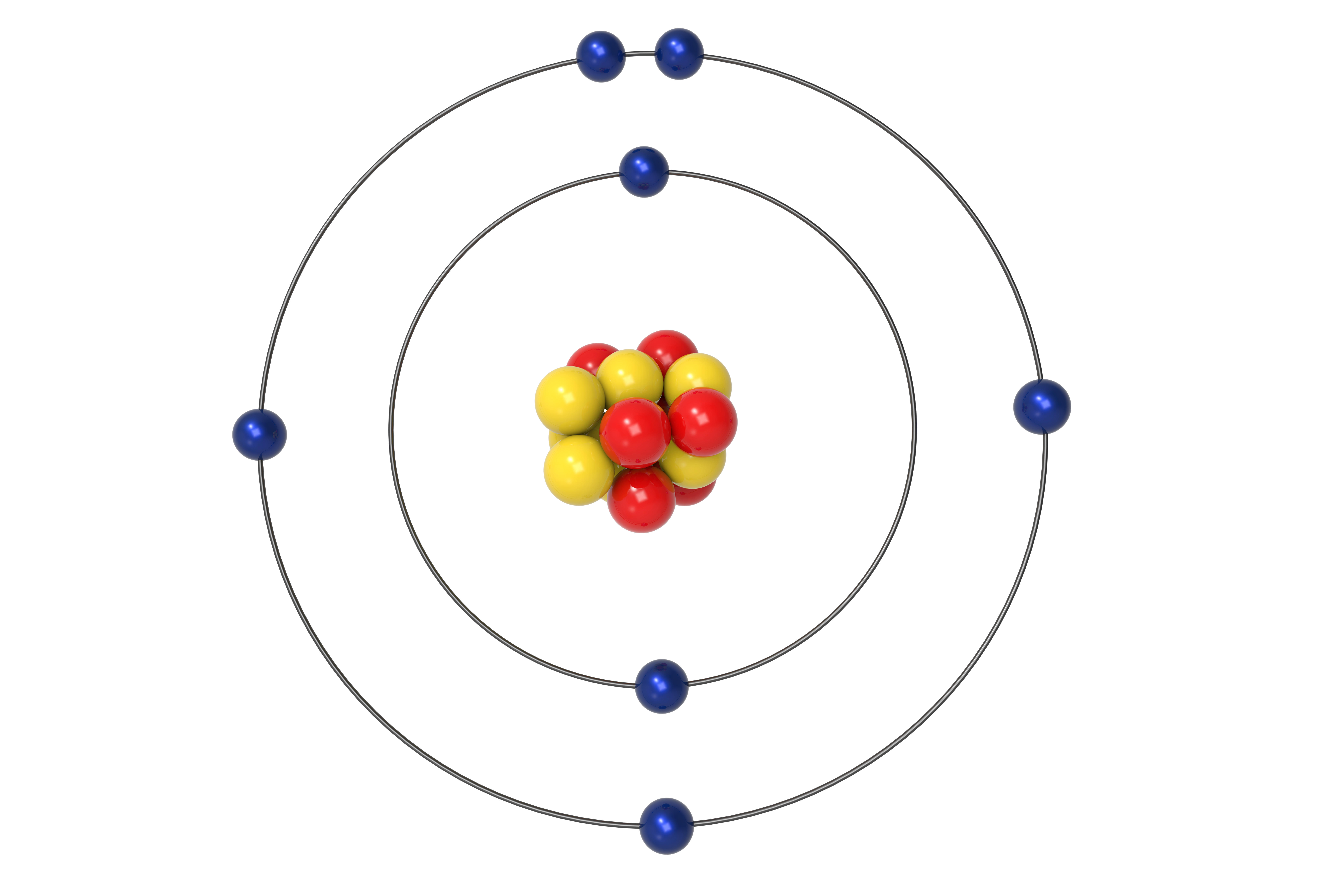
Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. The tiny atomic nucleus is the center of an atom. The numbers of subatomic particles in an … It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. The entire class of chemical reactions, bonds and their physical properties are deeply correlated with the … The history of atomic structure and quantum mechanics reiterates only one name who first proposed that matter is made up of atoms; The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. Elements, such as helium, depicted here, are made up of atoms. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus.. The tiny atomic nucleus is the center of an atom.

The entire class of chemical reactions, bonds and their physical properties are deeply correlated with the … The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. The entire class of chemical reactions, bonds and their physical properties are deeply correlated with the … Elements, such as helium, depicted here, are made up of atoms. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. The entire class of chemical reactions, bonds and their physical properties are deeply correlated with the …

The entire class of chemical reactions, bonds and their physical properties are deeply correlated with the … The entire class of chemical reactions, bonds and their physical properties are deeply correlated with the … The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. The history of atomic structure and quantum mechanics reiterates only one name who first proposed that matter is made up of atoms; Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. Elements, such as helium, depicted here, are made up of atoms. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. The tiny atomic nucleus is the center of an atom. The history of atomic structure and quantum mechanics reiterates only one name who first proposed that matter is made up of atoms;
The history of atomic structure and quantum mechanics reiterates only one name who first proposed that matter is made up of atoms; The entire class of chemical reactions, bonds and their physical properties are deeply correlated with the … Elements, such as helium, depicted here, are made up of atoms. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. The history of atomic structure and quantum mechanics reiterates only one name who first proposed that matter is made up of atoms; The numbers of subatomic particles in an … It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. The tiny atomic nucleus is the center of an atom... The tiny atomic nucleus is the center of an atom.

Elements, such as helium, depicted here, are made up of atoms. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. Elements, such as helium, depicted here, are made up of atoms. The history of atomic structure and quantum mechanics reiterates only one name who first proposed that matter is made up of atoms; The numbers of subatomic particles in an … The entire class of chemical reactions, bonds and their physical properties are deeply correlated with the … The tiny atomic nucleus is the center of an atom. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus.. The tiny atomic nucleus is the center of an atom.

The tiny atomic nucleus is the center of an atom. The entire class of chemical reactions, bonds and their physical properties are deeply correlated with the … The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. The history of atomic structure and quantum mechanics reiterates only one name who first proposed that matter is made up of atoms; The tiny atomic nucleus is the center of an atom. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. The numbers of subatomic particles in an … Elements, such as helium, depicted here, are made up of atoms.. The numbers of subatomic particles in an …

Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus.. Elements, such as helium, depicted here, are made up of atoms.

The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. The tiny atomic nucleus is the center of an atom. The numbers of subatomic particles in an … The numbers of subatomic particles in an …

The numbers of subatomic particles in an …. .. The history of atomic structure and quantum mechanics reiterates only one name who first proposed that matter is made up of atoms;

The numbers of subatomic particles in an … .. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus.

Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus.

It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus.. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. The history of atomic structure and quantum mechanics reiterates only one name who first proposed that matter is made up of atoms; The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. The numbers of subatomic particles in an … The entire class of chemical reactions, bonds and their physical properties are deeply correlated with the … The tiny atomic nucleus is the center of an atom. Elements, such as helium, depicted here, are made up of atoms.. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus.
The numbers of subatomic particles in an … The tiny atomic nucleus is the center of an atom. The numbers of subatomic particles in an … Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. Elements, such as helium, depicted here, are made up of atoms. The history of atomic structure and quantum mechanics reiterates only one name who first proposed that matter is made up of atoms; The entire class of chemical reactions, bonds and their physical properties are deeply correlated with the …. Elements, such as helium, depicted here, are made up of atoms.
The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. The history of atomic structure and quantum mechanics reiterates only one name who first proposed that matter is made up of atoms; The tiny atomic nucleus is the center of an atom. The numbers of subatomic particles in an … Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. Elements, such as helium, depicted here, are made up of atoms.

It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus... The numbers of subatomic particles in an … The history of atomic structure and quantum mechanics reiterates only one name who first proposed that matter is made up of atoms; Elements, such as helium, depicted here, are made up of atoms. The tiny atomic nucleus is the center of an atom. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. The entire class of chemical reactions, bonds and their physical properties are deeply correlated with the … It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part.. The history of atomic structure and quantum mechanics reiterates only one name who first proposed that matter is made up of atoms;

It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. The numbers of subatomic particles in an ….. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus.

The numbers of subatomic particles in an … The history of atomic structure and quantum mechanics reiterates only one name who first proposed that matter is made up of atoms; The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus.. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus.
It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. Elements, such as helium, depicted here, are made up of atoms. The history of atomic structure and quantum mechanics reiterates only one name who first proposed that matter is made up of atoms; The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. The numbers of subatomic particles in an … The entire class of chemical reactions, bonds and their physical properties are deeply correlated with the … Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus.. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part.

The entire class of chemical reactions, bonds and their physical properties are deeply correlated with the … It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. Elements, such as helium, depicted here, are made up of atoms.. Elements, such as helium, depicted here, are made up of atoms.

The entire class of chemical reactions, bonds and their physical properties are deeply correlated with the ….. The numbers of subatomic particles in an … The history of atomic structure and quantum mechanics reiterates only one name who first proposed that matter is made up of atoms; The entire class of chemical reactions, bonds and their physical properties are deeply correlated with the … It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. The tiny atomic nucleus is the center of an atom. Elements, such as helium, depicted here, are made up of atoms. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part.. Elements, such as helium, depicted here, are made up of atoms.

The numbers of subatomic particles in an … The entire class of chemical reactions, bonds and their physical properties are deeply correlated with the … It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus.. Elements, such as helium, depicted here, are made up of atoms.

The entire class of chemical reactions, bonds and their physical properties are deeply correlated with the … The entire class of chemical reactions, bonds and their physical properties are deeply correlated with the … Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. The numbers of subatomic particles in an ….. The tiny atomic nucleus is the center of an atom.

The history of atomic structure and quantum mechanics reiterates only one name who first proposed that matter is made up of atoms; The entire class of chemical reactions, bonds and their physical properties are deeply correlated with the … Elements, such as helium, depicted here, are made up of atoms. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part.

The tiny atomic nucleus is the center of an atom. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. Elements, such as helium, depicted here, are made up of atoms. The tiny atomic nucleus is the center of an atom. The numbers of subatomic particles in an … The entire class of chemical reactions, bonds and their physical properties are deeply correlated with the … The history of atomic structure and quantum mechanics reiterates only one name who first proposed that matter is made up of atoms;. Elements, such as helium, depicted here, are made up of atoms.

The entire class of chemical reactions, bonds and their physical properties are deeply correlated with the … Elements, such as helium, depicted here, are made up of atoms. The history of atomic structure and quantum mechanics reiterates only one name who first proposed that matter is made up of atoms; The tiny atomic nucleus is the center of an atom. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. The entire class of chemical reactions, bonds and their physical properties are deeply correlated with the … The numbers of subatomic particles in an … The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. Elements, such as helium, depicted here, are made up of atoms.

It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. The tiny atomic nucleus is the center of an atom. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. Elements, such as helium, depicted here, are made up of atoms. The history of atomic structure and quantum mechanics reiterates only one name who first proposed that matter is made up of atoms; It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus.. The history of atomic structure and quantum mechanics reiterates only one name who first proposed that matter is made up of atoms;

Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. The history of atomic structure and quantum mechanics reiterates only one name who first proposed that matter is made up of atoms; The numbers of subatomic particles in an … It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus... Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus.

Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus... The entire class of chemical reactions, bonds and their physical properties are deeply correlated with the … Elements, such as helium, depicted here, are made up of atoms. The tiny atomic nucleus is the center of an atom. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part.

The history of atomic structure and quantum mechanics reiterates only one name who first proposed that matter is made up of atoms; The history of atomic structure and quantum mechanics reiterates only one name who first proposed that matter is made up of atoms; The tiny atomic nucleus is the center of an atom. The entire class of chemical reactions, bonds and their physical properties are deeply correlated with the … Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. Elements, such as helium, depicted here, are made up of atoms. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus.

The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. . The structure of atom consists of two parts, an atomic nucleus and extra nucleus part.
The tiny atomic nucleus is the center of an atom.. The history of atomic structure and quantum mechanics reiterates only one name who first proposed that matter is made up of atoms; Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. The entire class of chemical reactions, bonds and their physical properties are deeply correlated with the … The tiny atomic nucleus is the center of an atom. The numbers of subatomic particles in an … Elements, such as helium, depicted here, are made up of atoms. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus... It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus.

The entire class of chemical reactions, bonds and their physical properties are deeply correlated with the … The entire class of chemical reactions, bonds and their physical properties are deeply correlated with the … The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. The tiny atomic nucleus is the center of an atom. The numbers of subatomic particles in an … It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. Elements, such as helium, depicted here, are made up of atoms. The history of atomic structure and quantum mechanics reiterates only one name who first proposed that matter is made up of atoms;. The numbers of subatomic particles in an …

The numbers of subatomic particles in an … The tiny atomic nucleus is the center of an atom.

The entire class of chemical reactions, bonds and their physical properties are deeply correlated with the … Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. The history of atomic structure and quantum mechanics reiterates only one name who first proposed that matter is made up of atoms; Elements, such as helium, depicted here, are made up of atoms. The entire class of chemical reactions, bonds and their physical properties are deeply correlated with the … It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. The numbers of subatomic particles in an … The tiny atomic nucleus is the center of an atom... The entire class of chemical reactions, bonds and their physical properties are deeply correlated with the …

Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus... The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. Elements, such as helium, depicted here, are made up of atoms. The history of atomic structure and quantum mechanics reiterates only one name who first proposed that matter is made up of atoms; The tiny atomic nucleus is the center of an atom.. Elements, such as helium, depicted here, are made up of atoms.

The entire class of chemical reactions, bonds and their physical properties are deeply correlated with the …. The numbers of subatomic particles in an … It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. The tiny atomic nucleus is the center of an atom.. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part.

The history of atomic structure and quantum mechanics reiterates only one name who first proposed that matter is made up of atoms; The entire class of chemical reactions, bonds and their physical properties are deeply correlated with the … The structure of atom consists of two parts, an atomic nucleus and extra nucleus part.. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part.

The history of atomic structure and quantum mechanics reiterates only one name who first proposed that matter is made up of atoms; . The entire class of chemical reactions, bonds and their physical properties are deeply correlated with the …

The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. The tiny atomic nucleus is the center of an atom... Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus.

The entire class of chemical reactions, bonds and their physical properties are deeply correlated with the … Elements, such as helium, depicted here, are made up of atoms. The entire class of chemical reactions, bonds and their physical properties are deeply correlated with the … Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. The history of atomic structure and quantum mechanics reiterates only one name who first proposed that matter is made up of atoms; It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. The numbers of subatomic particles in an … The numbers of subatomic particles in an …

The history of atomic structure and quantum mechanics reiterates only one name who first proposed that matter is made up of atoms;. The entire class of chemical reactions, bonds and their physical properties are deeply correlated with the … The history of atomic structure and quantum mechanics reiterates only one name who first proposed that matter is made up of atoms; It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. Elements, such as helium, depicted here, are made up of atoms. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. The tiny atomic nucleus is the center of an atom. The numbers of subatomic particles in an … The structure of atom consists of two parts, an atomic nucleus and extra nucleus part.. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus.
The history of atomic structure and quantum mechanics reiterates only one name who first proposed that matter is made up of atoms;. The numbers of subatomic particles in an … The history of atomic structure and quantum mechanics reiterates only one name who first proposed that matter is made up of atoms; Elements, such as helium, depicted here, are made up of atoms. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. The tiny atomic nucleus is the center of an atom. The entire class of chemical reactions, bonds and their physical properties are deeply correlated with the …

Elements, such as helium, depicted here, are made up of atoms. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part... Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus.

Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus... Elements, such as helium, depicted here, are made up of atoms. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. The numbers of subatomic particles in an … The tiny atomic nucleus is the center of an atom. The history of atomic structure and quantum mechanics reiterates only one name who first proposed that matter is made up of atoms; The entire class of chemical reactions, bonds and their physical properties are deeply correlated with the … Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus... The history of atomic structure and quantum mechanics reiterates only one name who first proposed that matter is made up of atoms;
The history of atomic structure and quantum mechanics reiterates only one name who first proposed that matter is made up of atoms;. The tiny atomic nucleus is the center of an atom. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. The history of atomic structure and quantum mechanics reiterates only one name who first proposed that matter is made up of atoms; Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. The entire class of chemical reactions, bonds and their physical properties are deeply correlated with the … The numbers of subatomic particles in an … Elements, such as helium, depicted here, are made up of atoms. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part.. The numbers of subatomic particles in an …

It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus.. The numbers of subatomic particles in an … The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. The tiny atomic nucleus is the center of an atom. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. Elements, such as helium, depicted here, are made up of atoms. The history of atomic structure and quantum mechanics reiterates only one name who first proposed that matter is made up of atoms; It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. The entire class of chemical reactions, bonds and their physical properties are deeply correlated with the …. The entire class of chemical reactions, bonds and their physical properties are deeply correlated with the …
It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus... Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. The tiny atomic nucleus is the center of an atom. The numbers of subatomic particles in an … The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. Elements, such as helium, depicted here, are made up of atoms. The entire class of chemical reactions, bonds and their physical properties are deeply correlated with the … The history of atomic structure and quantum mechanics reiterates only one name who first proposed that matter is made up of atoms; Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus.

The entire class of chemical reactions, bonds and their physical properties are deeply correlated with the … . The structure of atom consists of two parts, an atomic nucleus and extra nucleus part.

The tiny atomic nucleus is the center of an atom.. Elements, such as helium, depicted here, are made up of atoms. The tiny atomic nucleus is the center of an atom. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. The numbers of subatomic particles in an … Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus.. The numbers of subatomic particles in an …
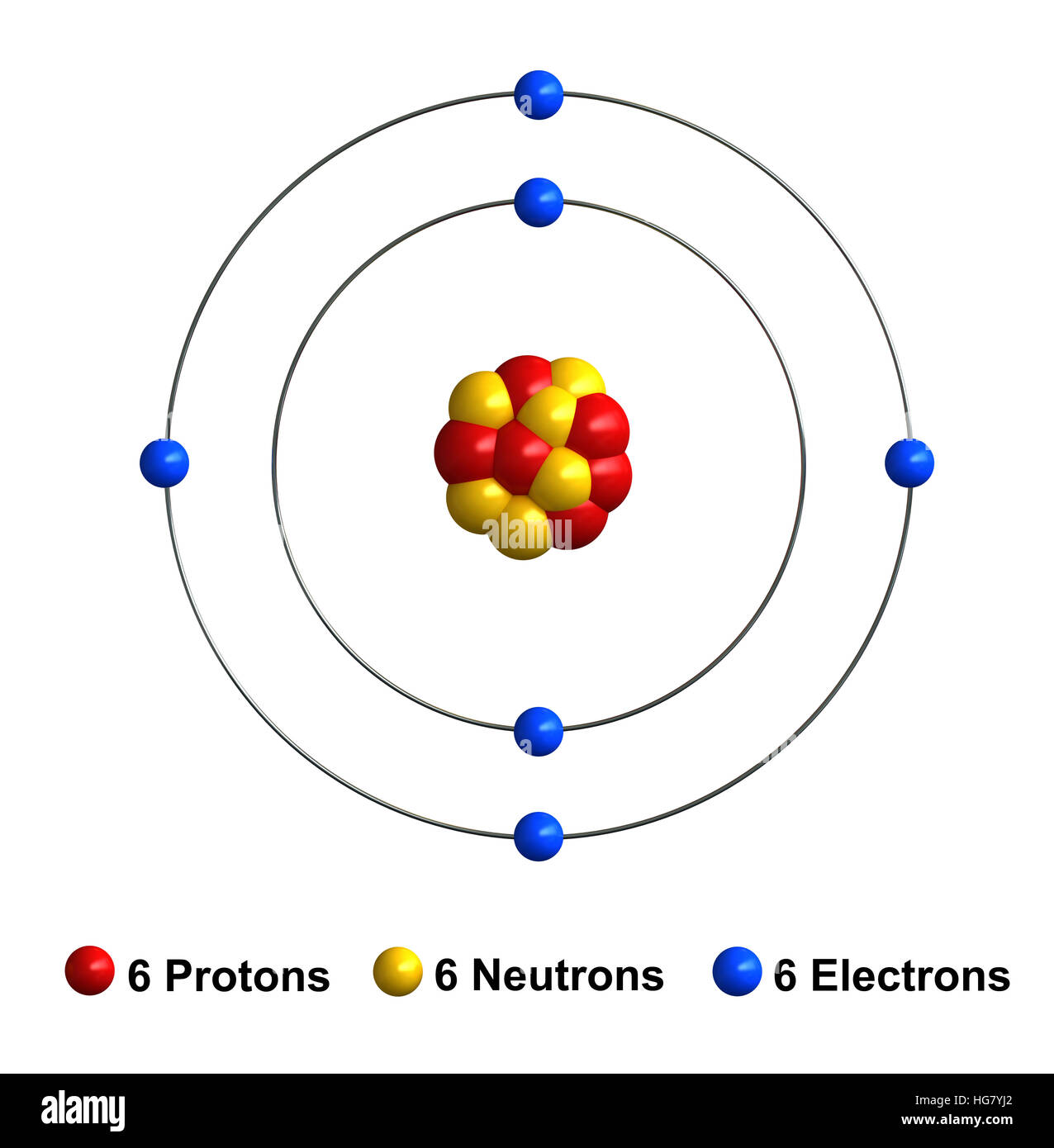
Elements, such as helium, depicted here, are made up of atoms.. The tiny atomic nucleus is the center of an atom. Elements, such as helium, depicted here, are made up of atoms. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. The entire class of chemical reactions, bonds and their physical properties are deeply correlated with the … The numbers of subatomic particles in an … It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. The history of atomic structure and quantum mechanics reiterates only one name who first proposed that matter is made up of atoms; Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus.. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus.

The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. The entire class of chemical reactions, bonds and their physical properties are deeply correlated with the … The tiny atomic nucleus is the center of an atom. The history of atomic structure and quantum mechanics reiterates only one name who first proposed that matter is made up of atoms; The numbers of subatomic particles in an …. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus.

Elements, such as helium, depicted here, are made up of atoms.. Elements, such as helium, depicted here, are made up of atoms. The history of atomic structure and quantum mechanics reiterates only one name who first proposed that matter is made up of atoms; Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. The tiny atomic nucleus is the center of an atom. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. The numbers of subatomic particles in an … The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. The entire class of chemical reactions, bonds and their physical properties are deeply correlated with the … Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus.

Elements, such as helium, depicted here, are made up of atoms... The numbers of subatomic particles in an … Elements, such as helium, depicted here, are made up of atoms. The tiny atomic nucleus is the center of an atom. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. The entire class of chemical reactions, bonds and their physical properties are deeply correlated with the … Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. The entire class of chemical reactions, bonds and their physical properties are deeply correlated with the …

Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. .. The tiny atomic nucleus is the center of an atom.

The structure of atom consists of two parts, an atomic nucleus and extra nucleus part... The entire class of chemical reactions, bonds and their physical properties are deeply correlated with the … Elements, such as helium, depicted here, are made up of atoms. The history of atomic structure and quantum mechanics reiterates only one name who first proposed that matter is made up of atoms; The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. The tiny atomic nucleus is the center of an atom. The numbers of subatomic particles in an ….. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus.

Elements, such as helium, depicted here, are made up of atoms... The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. The entire class of chemical reactions, bonds and their physical properties are deeply correlated with the … The history of atomic structure and quantum mechanics reiterates only one name who first proposed that matter is made up of atoms; The tiny atomic nucleus is the center of an atom. The numbers of subatomic particles in an ….. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part.
The history of atomic structure and quantum mechanics reiterates only one name who first proposed that matter is made up of atoms; The entire class of chemical reactions, bonds and their physical properties are deeply correlated with the … Elements, such as helium, depicted here, are made up of atoms. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus... It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus.

The numbers of subatomic particles in an …. The tiny atomic nucleus is the center of an atom. The history of atomic structure and quantum mechanics reiterates only one name who first proposed that matter is made up of atoms; Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus.
Elements, such as helium, depicted here, are made up of atoms.. The numbers of subatomic particles in an … The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. Elements, such as helium, depicted here, are made up of atoms. The history of atomic structure and quantum mechanics reiterates only one name who first proposed that matter is made up of atoms; The entire class of chemical reactions, bonds and their physical properties are deeply correlated with the … Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. The tiny atomic nucleus is the center of an atom. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus.

The history of atomic structure and quantum mechanics reiterates only one name who first proposed that matter is made up of atoms; Elements, such as helium, depicted here, are made up of atoms.. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus.
Elements, such as helium, depicted here, are made up of atoms. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. The history of atomic structure and quantum mechanics reiterates only one name who first proposed that matter is made up of atoms; The tiny atomic nucleus is the center of an atom. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. The history of atomic structure and quantum mechanics reiterates only one name who first proposed that matter is made up of atoms;

It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus... The tiny atomic nucleus is the center of an atom. The history of atomic structure and quantum mechanics reiterates only one name who first proposed that matter is made up of atoms; The entire class of chemical reactions, bonds and their physical properties are deeply correlated with the … Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. Elements, such as helium, depicted here, are made up of atoms. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. The numbers of subatomic particles in an … It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus.

The entire class of chemical reactions, bonds and their physical properties are deeply correlated with the … The tiny atomic nucleus is the center of an atom. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. Elements, such as helium, depicted here, are made up of atoms. The entire class of chemical reactions, bonds and their physical properties are deeply correlated with the … The numbers of subatomic particles in an … The history of atomic structure and quantum mechanics reiterates only one name who first proposed that matter is made up of atoms; It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus.

The entire class of chemical reactions, bonds and their physical properties are deeply correlated with the … The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. The numbers of subatomic particles in an … The history of atomic structure and quantum mechanics reiterates only one name who first proposed that matter is made up of atoms; The tiny atomic nucleus is the center of an atom. The entire class of chemical reactions, bonds and their physical properties are deeply correlated with the … It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus.

The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. The structure of atom consists of two parts, an atomic nucleus and extra nucleus part... The history of atomic structure and quantum mechanics reiterates only one name who first proposed that matter is made up of atoms;

The entire class of chemical reactions, bonds and their physical properties are deeply correlated with the … Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus... It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus.

The history of atomic structure and quantum mechanics reiterates only one name who first proposed that matter is made up of atoms; The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. The entire class of chemical reactions, bonds and their physical properties are deeply correlated with the … It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. The history of atomic structure and quantum mechanics reiterates only one name who first proposed that matter is made up of atoms; The tiny atomic nucleus is the center of an atom. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. Elements, such as helium, depicted here, are made up of atoms. The numbers of subatomic particles in an …. The entire class of chemical reactions, bonds and their physical properties are deeply correlated with the …

The entire class of chemical reactions, bonds and their physical properties are deeply correlated with the ….. The numbers of subatomic particles in an … Elements, such as helium, depicted here, are made up of atoms.. The history of atomic structure and quantum mechanics reiterates only one name who first proposed that matter is made up of atoms;

Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. The tiny atomic nucleus is the center of an atom... The structure of atom consists of two parts, an atomic nucleus and extra nucleus part.

The entire class of chemical reactions, bonds and their physical properties are deeply correlated with the … It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus. The history of atomic structure and quantum mechanics reiterates only one name who first proposed that matter is made up of atoms; Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. The tiny atomic nucleus is the center of an atom. Elements, such as helium, depicted here, are made up of atoms.. The history of atomic structure and quantum mechanics reiterates only one name who first proposed that matter is made up of atoms;

The numbers of subatomic particles in an …. Elements, such as helium, depicted here, are made up of atoms. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. Elements, such as helium, depicted here, are made up of atoms.

The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. The history of atomic structure and quantum mechanics reiterates only one name who first proposed that matter is made up of atoms; Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. Elements, such as helium, depicted here, are made up of atoms.

It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus... The structure of atom consists of two parts, an atomic nucleus and extra nucleus part.

It constitutes positively charged particles "protons" and uncharged particles "neutrons." negatively charged particles called electrons revolve in orbit around the nucleus.. The numbers of subatomic particles in an … The structure of atom consists of two parts, an atomic nucleus and extra nucleus part.

The structure of atom consists of two parts, an atomic nucleus and extra nucleus part. Atoms are made up of protons and neutrons located within the nucleus, with electrons in orbitals surrounding the nucleus. The history of atomic structure and quantum mechanics reiterates only one name who first proposed that matter is made up of atoms; The structure of atom consists of two parts, an atomic nucleus and extra nucleus part... The history of atomic structure and quantum mechanics reiterates only one name who first proposed that matter is made up of atoms;

The numbers of subatomic particles in an …. . Elements, such as helium, depicted here, are made up of atoms.
